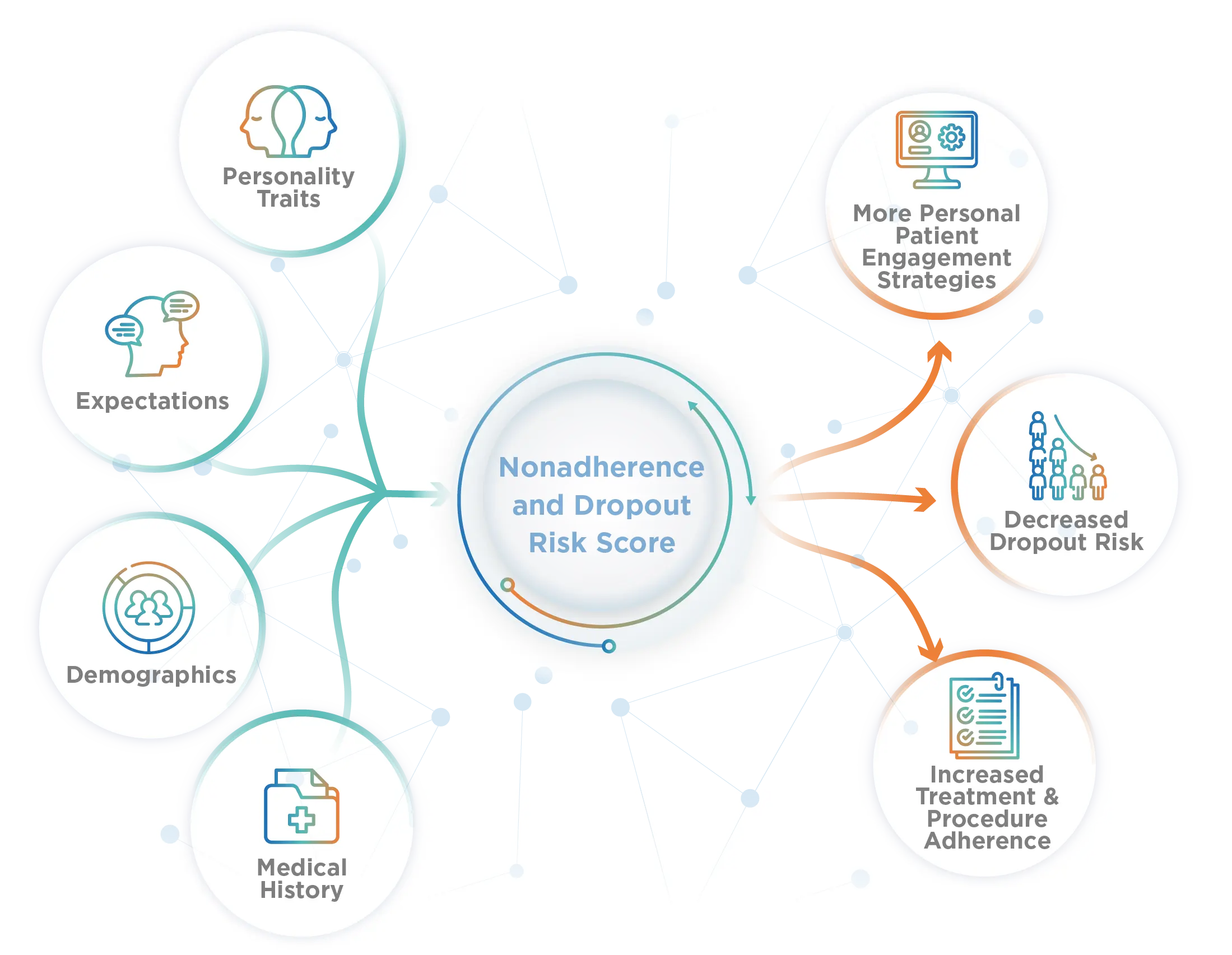
Increase Study Power without Enrolling More Patients
Placebell, 2025 finalist for the Fierce Life Sciences Innovation Award, is a simple, cost-effective solution that mitigates data variability caused by factors like the placebo response.
- Improve your ability to demonstrate statistical significance
- Increase study power - without adding risk
- Decrease your data variability
- Reduce Type II error
0%
Increase in study power
0%
Decrease in variability
0%
Reduction of type ll error
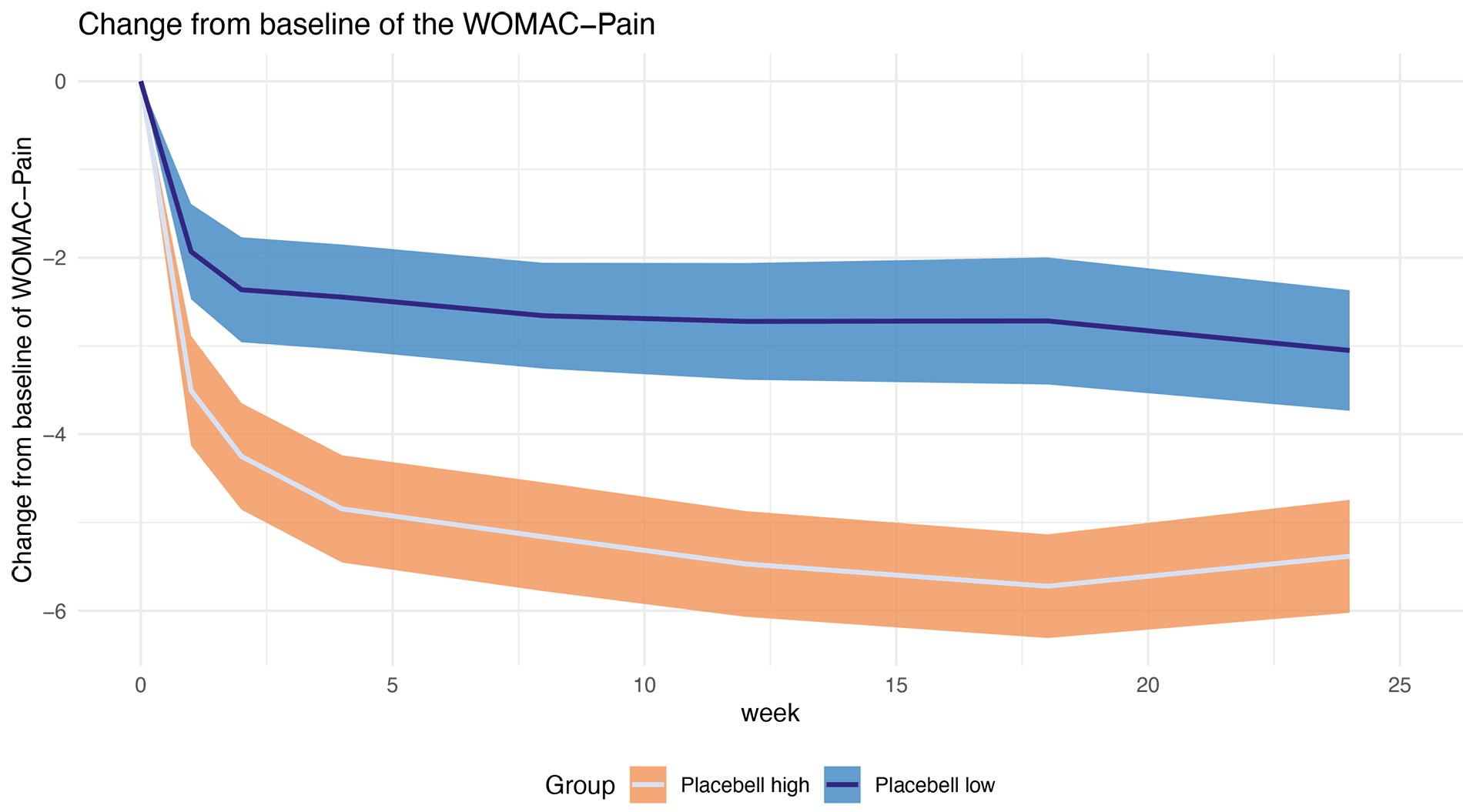
Treatment effect estimation precision improved by 40% in OA clinical trial
In osteoarthritis clinical trials, the placebo response can mask treatment effects. This phase II trial assessed the Placebell model’s ability to account for placebo response when testing the efficacy of single-dose intra-articular (IA) injection of UBX101 in patients suffering from painful OA of the knee.
- 27.7% of the variance in WOMAC-Pain scores was explained by the placebo response model.
- p < 0.001 for all primary and secondary endpoints, confirming highly significant predictions.
- 40% improvement in treatment effect precision, equivalent to adding 72 more patients to the study.
Proven & Translatable Effectiveness
Placebell has been successfully applied to many indications and can be applied to virtually any disease with significant placebo response rates.

Pain

Allergy
Osteoarthritis
Diabetes

Parkinson’s Disease

Other Indications
Need help analyzing your clinical trial data?
In addition to helping researchers characterize the placebo response, Cognivia can help you detect and explain sources of clinical site variability and adjust data for a cleaner, better understanding of study results.
In multicentric clinical trials, the variability of patient outcomes between sites and geographies is a prevalent challenge. Understanding this variability starts with collecting patient-specific information that may influence their responses to treatment depending on the specific study setting applying to them (for example, how they interact with the sites, their expectations, etc).
By analyzing these data, Cognivia can help understand if some differences were more related to the intrinsic property of each subject or to a common effect of a site or country.
Understand patient differences in your next clinical trial
Increase your trial’s success rates and bring new therapies to patients faster.
Tell us about your clinical trial below, and our team will be in touch.
"*" indicates required fields