Expert analytical services to characterize clinical sites
differences and response heterogeneity
In multicentric clinical trials, the variability of patient outcomes between sites and geographies is a prevalent challenge. The emergence of Decentralized Clinical Trials (or hybrid) offers many advantages - including enabling access to clinical trials to broader patient populations and improving patient engagement and adherence - but variability remains an overwhelming issue.
Understanding this variability starts with collecting patient-specific information that may influence their responses to treatment depending on the specific study setting applying to them (for example, how they interact with the sites, their expectations, etc).
In addition to our technology solutions, Cognivia offers expert analysis to help researchers detect and explain these sources of clinical "site" variability and adjust data for a cleaner and better understanding of the study's results.
Collect patients' insight and explain clinical data variability.
Step 1 Self-administer patient questionnaire at screening or baseline
Step 2 Evaluate clinical site and study setting heterogeneity at the end of the study
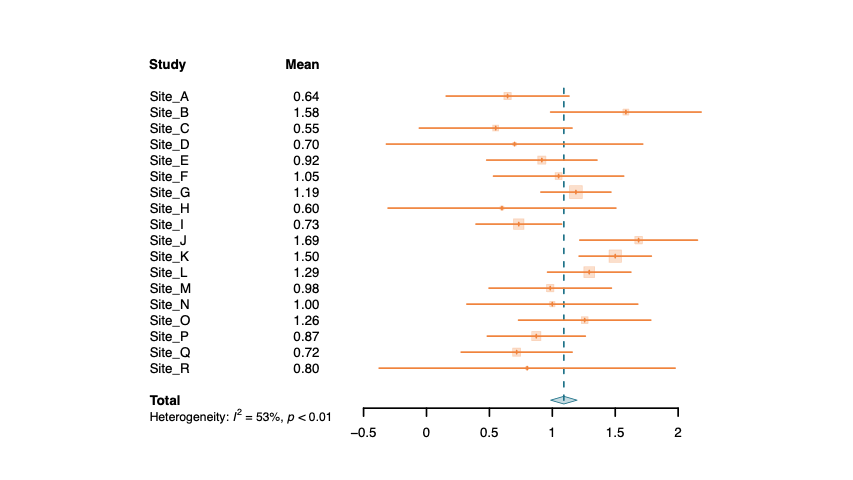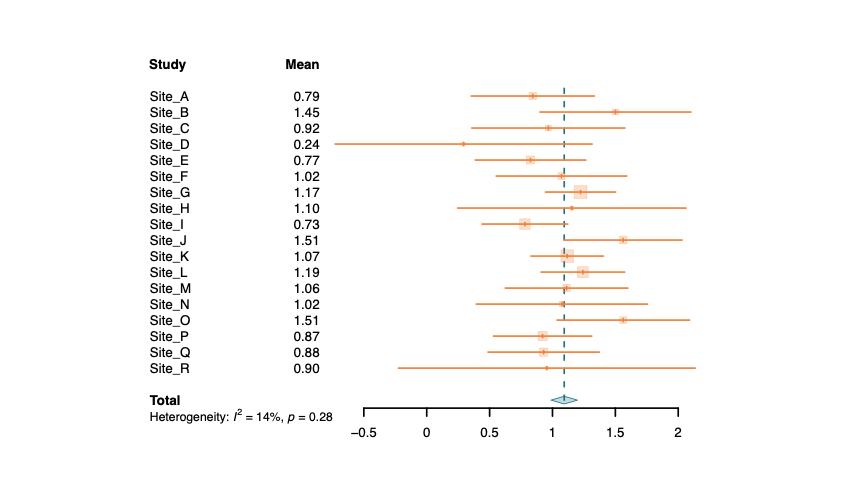
Variability in efficacy outcomes can be linked to the specific characteristics of each subject (psychological profile, beliefs, concerns, environment). A part of this variability can also come from a common effect of a country or site. Cognivia's questionnaires and supporting analyses can be used to better understand these differences. Using Cognivia's meta-features, it's possible to explain these differences and reduce the heterogeneity between sites in the statistical analysis. By analyzing these data, Cognivia can also help understand if some differences were more related to the intrinsic property of each subject or to a common effect of a site or country.
Examples of heterogeneity between sites with and without adjustment with patients' characteristics