While significant placebo responses rates are often noted in clinical trials for indications like pain and depression, this issue can plague drug development in any therapeutic area – particularly in diseases that rely on subjective or patient-reported outcomes as primary efficacy endpoints. Quality of life (QoL) endpoints, for example, are often used to measure therapeutic efficacy in oncology clinical trials – but also in diseases like schizophrenia, pain, heart failure, inflammatory bowel disease (IBD), allergy and pruritus. Significant placebo responses have been reported for QoL endpoints1,2, and efforts have been made (with only modest success) in determining factors that can predict which patients will be placebo responders for QoL3,4.
Similarly, sleep and fatigue may be either primary or secondary endpoints in a wide variety of indications. Sleep endpoints are used when evaluating drugs for insomnia, narcolepsy and obstructive sleep apnea, but are also used in clinical trials for Alzheimer’s, pain, psychiatric conditions and overactive bladder. Fatigue endpoints have been used to evaluate the efficacy of drugs for cancer, multiple sclerosis, Parkinson’s disease, rheumatoid arthritis and infectious diseases like influenza and COVID-19. Significant placebo responses have been reported in interventional clinical trials for sleep5,6 and fatigue7,8 endpoints. For example, placebo has been associated with significant reductions in patient-reported measures of sleep onset latency (SOL) and improvement in total sleep time (TST) and global sleep quality (GST) in patients with insonmia6.
Considering that the placebo response is in part responsible for the high rate of phase III clinical trial failures9, Cognivia has developed Placebell©™, which uses patient psychology data along with machine learning-based models to predict individual patients’ placebo response in a wide range of therapeutic areas and indications. These methods have recently been shown to improve clinical trial study power and assay sensitivity when used to define a baseline covariate accounting for the placebo response in the statistical analysis of clinical trial data10. Recently, Cognivia conducted a clinical trial in Parkinson’s disease (N=94) and, in this first trial, successfully explained the placebo response for the primary endpoint of the study, MDS-UPDRS part 311. This same approach was applied to other endpoints in the study, including Epworth Sleepiness Scale (ESS), Fatigue Severity Scale (FSS), and the Parkinson’s Disease Questionnaire (PDQ-39), a 39 item questionnaire that evaluates 8 specific areas (mobility, activities of daily living, emotional well-being, stigma, social support, cognitions, communications and bodily discomfort), yielding a single score that reflects disease-specific quality of life. This algorithm was able to significantly model placebo response for PDQ-39 and ESS, while the model of FSS was nearly statistically significant. This approach explained 11-23% of the data variability related to the placebo response in these endpoints (Table 1). Using the score calculated by this Placebell©™ model as a baseline covariate would result in a similar reduction in data variability of 11-23%.
Table 1.
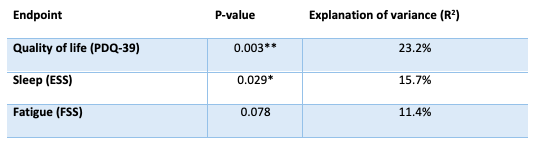
The Placebell©™ method significantly modeled the placebo response in quality of life, sleep and fatigue endpoints. R2 represents the strength of the correlation between the actual placebo response and the model. Using this information as a baseline covariate in statistical analyses results in a reduction of data variability equivalent to the R2.
The models developed through this study could be applied to any clinical trial evaluating quality of life, sleep or fatigue endpoints, while additional data would contribute to improvements to the algorithm. In this paradigm, the trained model could be pre-specified in the data analysis of a clinical trial as long as Cognivia’s Multi-dimensional Psychological Questionnaire (MPsQ) is included (single administration to all patients). A single score, the Placebell©™ Covariate, is then calculated for each patient to describe their expected placebo response and used as a baseline covariate as a low-risk option to increase study power and reduce the risk of trial failure. Data variability could be reduced by as much as 23% for quality-of-life endpoints. The impact of this method will continually improve as more data are accumulated. To learn more about how Placebell©™ could reduce risk in clinical trials, please contact us.
References:
- McRae C, Cherin E, Yamazaki TG, et al. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Archives of general psychiatry. 2004;61(4):412-420. doi:10.1001/ARCHPSYC.61.4.412
- Estevinho MM, Afonso J, Rosa I, et al. Placebo Effect on the Health-related Quality of Life of Inflammatory Bowel Disease Patients: A Systematic Review With Meta-analysis. Journal of Crohn’s and Colitis. 2018;12(10):1232-1244. doi:10.1093/ECCO-JCC/JJY100
- Talley NJ, Locke GR, Lahr BD, et al. Predictors of the placebo response in functional dyspepsia. Alimentary Pharmacology & Therapeutics. 2006;23(7):923-936. doi:10.1111/J.1365-2036.2006.02845.X
- Fulda S, Wetter TC. Where dopamine meets opioids: a meta-analysis of the placebo effect in restless legs syndrome treatment studies. Brain. 2008;131(4):902-917. doi:10.1093/BRAIN/AWM244
- Bélanger L, Vallières A, Ivers H, Moreau V, Lavigne G, Morin CM. Meta-analysis of sleep changes in control groups of insomnia treatment trials. Journal of Sleep Research. 2007;16(1):77-84. doi:10.1111/j.1365-2869.2007.00566.x
- Yeung V, Sharpe L, Glozier N, Hackett ML, Colagiuri B. A systematic review and meta-analysis of placebo versus no treatment for insomnia symptoms. Sleep medicine reviews. 2018;38:17-27. doi:10.1016/J.SMRV.2017.03.006
- Zhou ES, Hall KT, Michaud AL, Blackmon JE, Partridge AH, Recklitis CJ. Open-label placebo reduces fatigue in cancer survivors: a randomized trial. Supportive Care in Cancer. 2019;27(6):2179-2187. doi:10.1007/s00520-018-4477-6
- Wolters F, Peerdeman KJ, Evers AWM. Placebo and Nocebo Effects Across Symptoms: From Pain to Fatigue, Dyspnea, Nausea, and Itch. Frontiers in Psychiatry. 2019;10:470. doi:10.3389/fpsyt.2019.00470
- Dumitrescu TP, McCune J, Schmith V. Is Placebo Response Responsible for Many Phase III Failures? Clinical Pharmacology and Therapeutics. 2019;106(6):1151-1154. doi:10.1002/cpt.1632
- Branders S, Pereira A, Bernard G, Ernst M, Dananberg J, Albert A. Leveraging historical data to optimize the number of covariates and their explained variance in the analysis of randomized clinical trials.: https://doi.org/101177/09622802211065246. Published online December 13, 2021:096228022110652. doi:10.1177/09622802211065246
- Branders S, Rascol O, Garraux G, et al. Modeling of the Placebo Response in Parkinson’s Disease. In: Proceedings of the International Parkinson and Movement Disorders Society MDS Virtual Congress. ; 2021:369.




